Pediatric Neuroblastoma Workup: Laboratory Studies, Imaging Studies, Other Tests
Laboratory Studies
Any child with a presumed diagnosis of neuroblastoma or any other childhood cancer should be referred to a pediatric cancer center for proper care and evaluation. Laboratory studies should include the following:
- CBC count and differential (Anemia or other cytopenias suggest bone marrow involvement.)
- Urine collection for catecholamines (VMA/HVA) and UA
- A single sample or collected urine test for VMA/HVA is highly accurate in CLIA approved laboratories. Centers usually send samples to a specialty laboratory and/or perform a timed collection of urine.
- A urinary catecholamine level is considered to be elevated if it is 3 standard deviations higher than the age-related reference range levels.
- Serum creatinine
- Liver function tests
- Alanine aminotransferase (ALT)
- Aspartate aminotransferase (AST)
- Total bilirubin
- Alkaline phosphatase
- Total protein
- Albumin
- Prothrombin time (PT)/activated prothrombin time (aPTT)
- Electrolytes
- Calcium
- Magnesium
- Phosphorus
- Uric acid
- Serum lactate dehydrogenase (LDH)
- Ferritin
- Thyroid-stimulating hormone (TSH), T4
- Immunoglobulin (Ig)G levels
Imaging Studies
The following studies may be indicated in patients with neuroblastomas:
- Obtain chest and abdominal radiographs to evaluate for the presence of a posterior mediastinal mass or calcifications.
- A CT scan of the primary site is essential to determine tumor extent. The main body of the tumor is usually indistinguishable from nodal masses. See the images below.
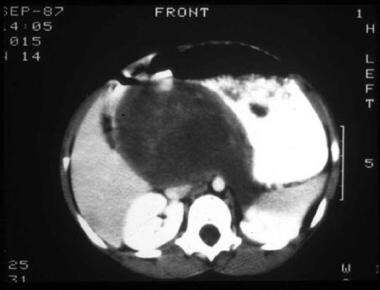 CT scan of abdomen in a patient with a retroperitoneal mass arising from the upper pole of the left kidney and elevated urine catecholamines.
CT scan of abdomen in a patient with a retroperitoneal mass arising from the upper pole of the left kidney and elevated urine catecholamines.  A one-week-old neonate had abdominal ultrasonography for evaluation of projectile vomiting. A right adrenal mass (100% cystic) was an incidental finding. Evaluation of the mass by CT was consistent with an adrenal bleed (3.6 x 3.1 x 2.4 cc). The infant was followed at 2 weeks (2-dimensional size diminished to 1.5 x. 2.4 cm2 on ultrasonography) and then at 6 weeks to document that the adrenal bleed continued to involute. Urine catecholamines were normal.
A one-week-old neonate had abdominal ultrasonography for evaluation of projectile vomiting. A right adrenal mass (100% cystic) was an incidental finding. Evaluation of the mass by CT was consistent with an adrenal bleed (3.6 x 3.1 x 2.4 cc). The infant was followed at 2 weeks (2-dimensional size diminished to 1.5 x. 2.4 cm2 on ultrasonography) and then at 6 weeks to document that the adrenal bleed continued to involute. Urine catecholamines were normal. - In cases of paraspinal masses, MRI aids in determining the presence of intraspinal tumor and cord compression. Horner syndrome should be evaluated with an MRI of the neck and head. See the image below.
 MRI of a left adrenal mass. The mass was revealed by fetal ultrasonography at 30 weeks' gestation. During infancy, the mass was found on the inferior pole of the left adrenal and was completely resected. Before surgery, the metastatic workup was negative. Surgical pathology service confirmed a diagnosis of neuroblastoma. After 3 years of follow-up care, no recurrence was observed.
MRI of a left adrenal mass. The mass was revealed by fetal ultrasonography at 30 weeks' gestation. During infancy, the mass was found on the inferior pole of the left adrenal and was completely resected. Before surgery, the metastatic workup was negative. Surgical pathology service confirmed a diagnosis of neuroblastoma. After 3 years of follow-up care, no recurrence was observed. - I -methyliodobenzylguanadine (MIBG) accumulates in catecholaminergic cells and provides a specific way of identifying primary and metastatic disease if present. Increasing numbers of institutions have access to MIBG scanning.
- A technetium-99 bone scan can also be used to evaluate bone metastases. This may be especially helpful in patients with negative MIBG study findings. Most current therapeutic protocols require both a bone scan and MIBG scan.
- Skeletal surveys may also be useful, especially in patients with multiple metastatic lesions.
- Positron emission tomography (PET) scan are under evaluation but are not currently recommended as part of the radiographic workup.
Other Tests
Obtain the following as baseline studies before therapy with anthracyclines:
- ECG
- Echocardiogram or resting radionuclide ejection fraction scan
Baseline hearing tests are recommended before cisplatin therapy. Baseline creatinine clearance should be measured (see the Creatinine Clearance (measured) calculator), especially if serum creatinine is abnormal.
Procedures
Bilateral bone marrow aspirates and biopsies should be performed to exclude metastatic disease.
Biopsy or resection of the primary tumor (stage I or II disease) is performed to collect tissue samples for biologic studies used to assign the patient into the appropriate risk category. Most centers in the United States perform limited open biopsies when the primary tumor is unresectable upfront. Adequate tissue is needed to perform molecular studies that aid in risk assignment. Extensive resections should be avoided upfront if they may place patient at excessive risk from morbidity or mortality from surgery. Neuroblastoma is a chemo-sensitive tumor; thus, second-look surgery to resect a residual primary may be a safer procedure with biopsy only performed upfront.
Tissue samples from a primary or metastatic tumor may be undifferentiated and confused with other small, round, blue cell tumors of childhood; however, immunohistochemical stains can aid with tissue diagnosis.
Molecular techniques, such as fluorescent in situ hybridization (FISH), can detect MYCN amplification, an important prognostic marker. Polymerase chain reaction (PCR) can identify specific translocations, such as t(11;22), in Ewing sarcoma and t(2;13) in alveolar rhabdomyosarcoma, thus ruling out neuroblastoma.
Neuroblastoma in bone marrow can be difficult to distinguish from other small, round, blue cell tumors of childhood.
Histologic Findings
Biopsy findings are usually required to diagnose neuroblastoma. Depending on the extent of disease at presentation, consider complete surgical resection, especially in patients with low-stage disease. Even without a biopsy, the presence of elevated urinary catecholamines and a bone marrow aspirate or biopsy with unequivocal neuroblastoma cells is diagnostic.
Histologically, neural crest tumors can be classified as neuroblastoma, ganglioneuroblastoma, and ganglioneuroma, depending on the degree of maturation and differentiation of the tumor. Undifferentiated neuroblastomas histologically present as small, round, blue cell tumors with dense nests of cells in a fibrovascular matrix and Homer-Wright pseudorosettes. These pseudorosettes, observed in 15-50% of tumor samples can be described as neuroblasts surrounding eosinophilic neuritic processes. The typical tumor shows small uniform cells with scant cytoplasm and hyperchromatic nuclei. A neuritic process, also called neuropil, is a pathognomonic feature of neuroblastoma.
Neuron-specific enolase (NSE), chromogranin, synaptophysin, and S-100 immunohistochemical stain findings are usually positive. Electron microscopy can be useful because ultrastructural features (eg, neurofilaments, neurotubules, synaptic vessels, dense core granules) are diagnostic for neuroblastoma. In contrast, the completely benign ganglioneuroma is typically composed of mature ganglion cells, Schwann cells, and neuritic processes, whereas ganglioneuroblastomas include the whole spectrum of differentiation between pure ganglioneuromas and neuroblastomas.
The pathologist must thoroughly evaluate the tumor because regions with different gross appearance may exhibit a different histology.
Staging
The patient should undergo a staging workup along with surgical resection or biopsy, as appropriate. Using various molecular features in conjunction with pathology and staging is essential to appropriately stratify patients and determine the best therapy.
The International Neuroblastoma Staging System (INSS) is currently used in all cooperative group studies in the United States. Recently, the International Neuroblastoma Risk Group Staging System (INRGSS) and International Neuroblastoma Risk Group Consensus Pretreatment Classification were released.The current INSS system is based on degree of surgical resection and thus is not appropriate for use with the INRG Pretreatment Classification. This is especially important because not all groups use upfront surgical resection as part of their staging system. The INRG was formulated to be used in international settings and to facilitate comparison of treatment outcomes across studies to allow common definitions among all groups. Thus, development of the INRGSS was facilitated using pretreatment tumor imaging rather than extent of surgical resection.
The INRGSS is as follows:
- L1 - Localized tumor not involving vital structures, as defined by the list of image-defined risk factors and confined to one body component
- L2 - Locoregional tumor with presence of one or more image-defined risk factors
- M - Distant metastatic disease
- MS - Metastatic disease in children younger than 18 months with metastases confined to skin, liver, and/or bone marrow
The INSS is as follows:
- Stage 1
- Localized tumor with complete gross excision, microscopic residual disease, or both
- Ipsilateral lymph nodes negative for tumor (Nodes attached to the primary tumor may be positive for tumor).
- Stage 2A
- Localized tumor with incomplete gross resection
- Representative ipsilateral nonadherent lymph nodes microscopically negative for tumor
- Stage 2B
- Localized tumor, complete gross excision, or both with ipsilateral nonadherent lymph nodes positive for tumor
- Enlarged contralateral lymph nodes, which are negative for tumor microscopically
- Stage 3
- Unresectable unilateral tumor infiltrating across the midline, regional lymph node involvement, or both
- Alternatively, localized unilateral tumor with contralateral regional lymph node involvement
- Stage 4 - Any primary tumor with dissemination to distant lymph nodes, bone, bone marrow, liver, skin, and/or other organs (except as defined for stage 4S)
- Stage 4S
- Localized primary tumor (as defined for stages 1, 2A, or 2B) with dissemination limited to skin, liver, and/or bone marrow (< 10% involvement)
- Limited to infants
Treatment & Management
Norman J Lacayo, MD Assistant Professor, Department of Pediatrics, Division of Hematology-Oncology, Stanford University and Lucile Salter Packard Children's Hospital
Norman J Lacayo, MD is a member of the following medical societies: Alpha Omega Alpha, American Society of Hematology, Children's Oncology Group
Coauthor(s)
Kara L Davis, DO Instructor, Department of Pediatric Hematology/Oncology, Stanford University School of Medicine
Kara L Davis, DO is a member of the following medical societies: American Society of Hematology
Specialty Editor Board
Mary L Windle, PharmD Adjunct Associate Professor, University of Nebraska Medical Center College of Pharmacy; Editor-in-Chief, Medscape Drug Reference
Steven K Bergstrom, MD Department of Pediatrics, Division of Hematology-Oncology, Kaiser Permanente Medical Center of Oakland
Steven K Bergstrom, MD is a member of the following medical societies: Alpha Omega Alpha, Children's Oncology Group, American Society of Clinical Oncology, International Society for Experimental Hematology, American Society of Hematology, American Society of Pediatric Hematology/Oncology
Chief Editor
Max J Coppes, MD, PhD, MBA Executive Vice President, Chief Medical and Academic Officer, Renown Heath
Max J Coppes, MD, PhD, MBA is a member of the following medical societies: American College of Healthcare Executives, American Society of Pediatric Hematology/Oncology, Society for Pediatric Research
Stephan A Grupp, MD, PhD Director, Stem Cell Biology Program, Department of Pediatrics, Division of Oncology, Children's Hospital of Philadelphia; Associate Professor of Pediatrics, University of Pennsylvania School of Medicine
Stephan A Grupp, MD, PhD is a member of the following medical societies: American Association for Cancer Research, Society for Pediatric Research, American Society for Blood and Marrow Transplantation, American Society of Hematology, American Society of Pediatric Hematology/Oncology
References
- Look AT, Hayes FA, Shuster JJ, et al. Clinical relevance of tumor cell ploidy and N-myc gene amplification in childhood neuroblastoma: a Pediatric Oncology Group study. J Clin Oncol. 1991 Apr. 9(4):581-91. [Medline].
- [Guideline] Shimada H, Chatten J, Newton WA Jr, et al. Histopathologic prognostic factors in neuroblastic tumors: definition of subtypes of ganglioneuroblastoma and an age-linked classification of neuroblastomas. J Natl Cancer Inst. 1984 Aug. 73(2):405-16. [Medline].
- [Guideline] Cohn SL, Pearson AD, London WB, et al. The International Neuroblastoma Risk Group (INRG) classification system: an INRG Task Force report. J Clin Oncol. 2009 Jan 10. 27(2):289-97. [Medline]. [Full Text].
- National Cancer Institute. SEER Pediatric Monograph. National Cancer Institute. Available at http://www-seer.ims.nci.nih.gov/Publications/PedMono/sympathetic.pdf. Accessed: February 25, 2002.
- Stiller CA, Parkin DM. International Variations in the incidence of neuroblastoma. International Journal of Cancer. 1992. 52:538-543.
- Haupt R, Garaventa A, Gambini C, et al. Improved Survival of Children with Neuroblastoma Between 1979 and 2005: A Report of the Italian Neuroblastoma Registry. J Clin Oncol. 2010 Mar 29. [Medline].
- George RE, Li S, Medeiros-Nancarrow C, et al. High-risk neuroblastoma treated with tandem autologous peripheral-blood stem cell-supported transplantation: long-term survival update. J Clin Oncol. 2006 Jun 20. 24(18):2891-6. [Medline].
- Mosse YP, Laudenslager M, Longo L, Cole KA, Wood A, Attiyeh EF. Identification of ALK as a major familial neuroblastoma predisposition gene. Nature. 2008 Oct 16. 455(7215):930-5. [Medline]. [Full Text].
- Schleiermacher G, Javanmardi N, Bernard V, et al. Emergence of New ALK Mutations at Relapse of Neuroblastoma. J Clin Oncol. 2014 Sep 1. 32(25):2727-34. [Medline].
- De Brouwer S, De Preter K, Kumps C, Zabrocki P, Porcu M, Westerhout EM. Meta-analysis of neuroblastomas reveals a skewed ALK mutation spectrum in tumors with MYCN amplification. Clin Cancer Res. 2010 Sep 1. 16(17):4353-62. [Medline].
- Capasso M, Devoto M, Hou C, et al. Common variations in BARD1 influence susceptibility to high-risk neuroblastoma. Nat Genet. 2009 Jun. 41(6):718-23. [Medline]. [Full Text].
- Diskin SJ, Hou C, Glessner JT, Attiyeh EF, Laudenslager M, Bosse K. Copy number variation at 1q21.1 associated with neuroblastoma. Nature. 2009 Jun 18. 459(7249):987-91. [Medline].
- Wang K, Diskin SJ, Zhang H, Attiyeh EF, Winter C, Hou C. Integrative genomics identifies LMO1 as a neuroblastoma oncogene. Nature. 2011 Jan 13. 469(7329):216-20. [Medline].
- Nguyen le B, Diskin SJ, Capasso M, Wang K, Diamond MA, Glessner J. Phenotype restricted genome-wide association study using a gene-centric approach identifies three low-risk neuroblastoma susceptibility Loci. PLoS Genet. 2011 Mar. 7(3):e1002026. [Medline].
- [Guideline] Monclair T, Brodeur GM, Ambros PF, et al. The International Neuroblastoma Risk Group (INRG) staging system: an INRG Task Force report. J Clin Oncol. 2009 Jan 10. 27(2):298-303. [Medline]. [Full Text].
- London WB, Castleberry RP, Matthay KK, et al. Evidence for an age cutoff greater than 365 days for neuroblastoma risk group stratification in the Children's Oncology Group. J Clin Oncol. 2005 Sep 20. 23(27):6459-65. [Medline].
- London WB, Castleberry RP, Matthay KK, Look AT, Seeger RC, Shimada H. Evidence for an age cutoff greater than 365 days for neuroblastoma risk group stratification in the Children's Oncology Group. J Clin Oncol. 2005 Sep 20. 23(27):6459-65. [Medline].
- Maris JM, Hogarty MD, Bagatell R, Cohn SL. Neuroblastoma. Lancet. 2007 Jun 23. 369(9579):2106-20. [Medline].
- Strother DR, London WB, Schmidt ML, et al. Outcome After Surgery Alone or With Restricted Use of Chemotherapy for Patients With Low-Risk Neuroblastoma: Results of Children's Oncology Group Study P9641. J Clin Oncol. 2012 May 20. 30(15):1842-8. [Medline].
- Baker DL, Schmidt ML, Cohn SL, et al. Outcome after reduced chemotherapy for intermediate-risk neuroblastoma. N Engl J Med. 2010 Sep 30. 363(14):1313-23. [Medline].
- Yu AL, Gilman AL, Ozkaynak MF, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010 Sep 30. 363(14):1324-34. [Medline].
- Naranjo A, Parisi MT, Shulkin BL, et al. Comparison of ¹²³I-metaiodobenzylguanidine (MIBG) and ¹³¹I-MIBG semi-quantitative scores in predicting survival in patients with stage 4 neuroblastoma: a report from the Children's Oncology Group. Pediatr Blood Cancer. 2011 Jul 1. 56(7):1041-5. [Medline].
- Mueller S, Yang X, Sottero TL, Gragg A, Prasad G, Polley MY. Cooperation of the HDAC inhibitor vorinostat and radiation in metastatic neuroblastoma: efficacy and underlying mechanisms. Cancer Lett. 2011 Jul 28. 306(2):223-9. [Medline].
- Ladenstein R, Valteau-Couanet D, Brock P, et al. Randomized Trial of prophylactic granulocyte colony-stimulating factor during rapid COJEC induction in pediatric patients with high-risk neuroblastoma: the European HR-NBL1/SIOPEN study. J Clin Oncol. 2010 Jul 20. 28(21):3516-24. [Medline].
- Acharya S, Jayabose S, Kogan SJ, et al. Prenatally diagnosed neuroblastoma. Cancer. 1997 Jul 15. 80(2):304-10. [Medline].
- Altman AJ. Management of malignant solid tumors. Hematology of Infancy and Childhood. 1993.
- Attiyeh EF, London WB, Mosse YP, et al. Chromosome 1p and 11q deletions and outcome in neuroblastoma. N Engl J Med. 2005 Nov 24. 353(21):2243-53. [Medline].
- Bagatell R, Rumcheva P, London WB, et al. Outcomes of children with intermediate-risk neuroblastoma after treatment stratified by MYCN status and tumor cell ploidy. J Clin Oncol. 2005 Dec 1. 23(34):8819-27. [Medline].
- Berthold F, Baillot A, Hero B, et al. Which cases are found and missed by neuroblastoma screening at 1 year? Results from the 1992 to 1995 study in three Federal States of Germany. J Clin Oncol. 1999 Apr. 17(4):1200. [Medline].
- Bowman LC, Castleberry RP, Cantor A, et al. Genetic staging of unresectable or metastatic neuroblastoma in infants: a Pediatric Oncology Group study. J Natl Cancer Inst. 1997 Mar 5. 89(5):373-80. [Medline].
- Brodeur GM. Molecular pathology of human neuroblastomas. Semin Diagn Pathol. 1994 May. 11(2):118-25. [Medline].
- Brodeur GM, Castleberry RP. Neuroblastoma. Pizzo PA, Poplack DG, eds. Principles and Practices of Pediatric Oncology. 2006. 933.
- Brodeur GM, Nakagawara A, Yamashiro DJ, et al. Expression of TrkA, TrkB and TrkC in human neuroblastomas. J Neurooncol. 1997 Jan. 31(1-2):49-55. [Medline].
- Brodeur GM, Pritchard J, Berthold F, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol. 1993 Aug. 11(8):1466-77. [Medline].
- Brodeur GM, Seeger RC, Schwab M, et al. Amplification of N-myc in untreated human neuroblastomas correlates with advanced disease stage. Science. 1984 Jun 8. 224(4653):1121-4. [Medline].
- Cachat F, Guignard JP. [The kidney in children under chemotherapy]. Rev Med Suisse Romande. 1996 Dec. 116(12):985-93. [Medline].
- Cachat F, Nenadov-Beck M, Guignard JP. Occurrence of an acute Fanconi syndrome following cisplatin chemotherapy. Med Pediatr Oncol. 1998 Jul. 31(1):40-1. [Medline].
- Carlsen NL. Neuroblastoma: epidemiology and pattern of regression. Problems in interpreting results of mass screening. Am J Pediatr Hematol Oncol. 1992 May. 14(2):103-10. [Medline].
- Castleberry RP. Predicting outcome in neuroblastoma. N Engl J Med. 1999 Jun 24. 340(25):1992-3. [Medline].
- Castleberry RP, Kun LE, Shuster JJ, et al. Radiotherapy improves the outlook for patients older than 1 year with Pediatric Oncology Group stage C neuroblastoma. J Clin Oncol. 1991 May. 9(5):789-95. [Medline].
- Chan HS, Grogan TM, DeBoer G, et al. Diagnosis and reversal of multidrug resistance in paediatric cancers. Eur J Cancer. 1996 Jun. 32A(6):1051-61. [Medline].
- Chatten J, Shimada H, Sather HN, et al. Prognostic value of histopathology in advanced neuroblastoma: a report from the Childrens Cancer Study Group. Hum Pathol. 1988 Oct. 19(10):1187-98. [Medline].
- Combaret V, Gross N, Lasset C, et al. Clinical relevance of CD44 cell surface expression and MYCN gene amplification in neuroblastoma. Eur J Cancer. 1997 Oct. 33(12):2101-5. [Medline].
- De Moerloose B, Dhooge C, Laureys G, et al. Discrepant flow cytometric expression and function of P-glycoprotein in neuroblastic tumors. Cytometry. 1999 Oct 1. 37(2):125-32. [Medline].
- Dockhorn-Dworniczak B, Schafer KL, Dantcheva R, et al. [Detection of EWS-/FLI-1 gene fusion transcripts by RT-PCR as a tool in the diagnosis of tumors of the Ewing sarcoma group]. Verh Dtsch Ges Pathol. 1994. 78:214-9. [Medline].
- Donovan J, Temel J, Zuckerman A, et al. CD34 selection as a stem cell purging strategy for neuroblastoma: preclinical and clinical studies. Med Pediatr Oncol. 2000 Dec. 35(6):677-82. [Medline].
- Downing JR, Khandekar A, Shurtleff SA, et al. Multiplex RT-PCR assay for the differential diagnosis of alveolar rhabdomyosarcoma and Ewing's sarcoma. Am J Pathol. 1995 Mar. 146(3):626-34. [Medline].
- Evans AR, Brand W, de Lorimier A, et al. Results in children with local and regional neuroblastoma managed with and without vincristine, cyclophosphamide, and imidazolecarboxamide. A report from the Children's Cancer Study Group. Am J Clin Oncol. 1984 Feb. 7(1):3-7. [Medline].
- Finklestein JZ. Neuroblastoma: the challenge and frustration. Hematol Oncol Clin North Am. 1987 Dec. 1(4):675-94. [Medline].
- Fish JD, Grupp SA. Stem cell transplantation for neuroblastoma. Bone Marrow Transplant. 2008 Jan. 41(2):159-65. [Medline].
- George RE, London WB, Cohn SL, et al. Hyperdiploidy plus nonamplified MYCN confers a favorable prognosis in children 12 to 18 months old with disseminated neuroblastoma: a Pediatric Oncology Group study. J Clin Oncol. 2005 Sep 20. 23(27):6466-73. [Medline].
- George RE, London WB, Cohn SL, et al. Hyperdiploidy plus nonamplified MYCN confers a favorable prognosis in children 12 to 18 months old with disseminated neuroblastoma: a Pediatric Oncology Group study. J Clin Oncol. 2005 Sep 20. 23(27):6466-73. [Medline].
- Gilbert F. Solid tumors of children: chromosome abnormalities and the development of cancer. J Cell Physiol Suppl. 1984. 3:165-70. [Medline].
- Grenier MA, Lipshultz SE. Epidemiology of anthracycline cardiotoxicity in children and adults. Semin Oncol. 1998 Aug. 25(4 Suppl 10):72-85. [Medline].
- Grupp SA, Stern JW, Bunin N, et al. Tandem high-dose therapy in rapid sequence for children with high-risk neuroblastoma. J Clin Oncol. 2000 Jul. 18(13):2567-75. [Medline].
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000 Jan 7. 100(1):57-70. [Medline].
- Hann HW, Evans AE, Siegel SE, et al. Prognostic importance of serum ferritin in patients with Stages III and IV neuroblastoma: the Childrens Cancer Study Group experience. Cancer Res. 1985 Jun. 45(6):2843-8. [Medline].
- Helson L. Neuroblastoma spinal cord compression (review). Anticancer Res. 1983 Sep-Oct. 3(5):317-22. [Medline].
- Holgersen LO, Santulli TV, Schullinger JN, Berdon WE. Neuroblastoma with intraspinal (dumbbell) extension. J Pediatr Surg. 1983 Aug. 18(4):406-11. [Medline].
- Hsiao RJ, Seeger RC, Yu AL, O'Connor DT. Chromogranin A in children with neuroblastoma. Serum concentration parallels disease stage and predicts survival. J Clin Invest. 1990 May. 85(5):1555-9. [Medline].
- Joshi VV, Cantor AB, Altshuler G, et al. Age-linked prognostic categorization based on a new histologic grading system of neuroblastomas. A clinicopathologic study of 211 cases from the Pediatric Oncology Group. Cancer. 1992 Apr 15. 69(8):2197-211. [Medline].
- Joshi VV, Cantor AB, Brodeur GM, et al. Correlation between morphologic and other prognostic markers of neuroblastoma. A study of histologic grade, DNA index, N-myc gene copy number, and lactic dehydrogenase in patients in the Pediatric Oncology Group. Cancer. 1993 May 15. 71(10):3173-81. [Medline].
- Joshi VV, Rao PV, Cantor AB, et al. Modified histologic grading of neuroblastomas by replacement of mitotic rate with mitosis karyorrhexis index. A clinicopathologic study of 223 cases from the Pediatric Oncology Group. Cancer. 1996 Apr 15. 77(8):1582-8. [Medline].
- LaBrosse EH, Com-Nougue C, Zucker JM, et al. Urinary excretion of 3-methoxy-4-hydroxymandelic acid and 3-methoxy-4- hydroxyphenylacetic acid by 288 patients with neuroblastoma and related neural crest tumors. Cancer Res. 1980 Jun. 40(6):1995-2001. [Medline].
- Laug WE, Siegel SE, Shaw KN, et al. Initial urinary catecholamine metabolite concentrations and prognosis in neuroblastoma. Pediatrics. 1978 Jul. 62(1):77-83. [Medline].
- Loebstein R, Atanackovic G, Bishai R, et al. Risk factors for long-term outcome of ifosfamide-induced nephrotoxicity in children. J Clin Pharmacol. 1999 May. 39(5):454-61. [Medline].
- Lucky AW, McGuire J, Komp DM. Infantile neuroblastoma presenting with cutaneous blanching nodules. J Am Acad Dermatol. 1982 Mar. 6(3):389-91. [Medline].
- Maitra A, Yashima K, Rathi A, et al. The RNA component of telomerase as a marker of biologic potential and clinical outcome in childhood neuroblastic tumors. Cancer. 1999 Feb 1. 85(3):741-9. [Medline].
- Maris JM. Unholy matrimony: Aurora A and N-Myc as malignant partners in neuroblastoma. Cancer Cell. 2009 Jan 6. 15(1):5-6. [Medline].
- Maris JM, White PS, Beltinger CP, et al. Significance of chromosome 1p loss of heterozygosity in neuroblastoma. Cancer Res. 1995 Oct 15. 55(20):4664-9. [Medline].
- Massaron S, Seregni E, Luksch R, et al. Neuron-specific enolase evaluation in patients with neuroblastoma. Tumour Biol. 1998. 19(4):261-8. [Medline].
- Matthay KK, Villablanca JG, Seeger RC, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children's Cancer Group. N Engl J Med. 1999 Oct 14. 341(16):1165-73. [Medline].
- McLeod HL, Relling MV, Crom WR, et al. Disposition of antineoplastic agents in the very young child. Br J Cancer Suppl. 1992 Aug. 18:S23-9. [Medline].
- Nitschke R, Smith EI, Altshuler G, et al. Postoperative treatment of nonmetastatic visible residual neuroblastoma: a Pediatric Oncology Group study. J Clin Oncol. 1991 Jul. 9(7):1181-8. [Medline].
- Nitschke R, Smith EI, Shochat S, et al. Localized neuroblastoma treated by surgery: a Pediatric Oncology Group Study. J Clin Oncol. 1988 Aug. 6(8):1271-9. [Medline].
- Norris MD, Bordow SB, Marshall GM, et al. Expression of the gene for multidrug-resistance-associated protein and outcome in patients with neuroblastoma. N Engl J Med. 1996 Jan 25. 334(4):231-8. [Medline].
- Parker L, Powell J. Screening for neuroblastoma in infants younger than 1 year of age. Review of the first 30 years. Medical Pediatric Oncology. 1998. 31:455-69.
- Perez CA, Matthay KK, Atkinson JB, Seeger RC, Shimada H, Haase GM. Biologic variables in the outcome of stages I and II neuroblastoma treated with surgery as primary therapy: a children's cancer group study. J Clin Oncol. 2000 Jan. 18(1):18-26. [Medline].
- Peuchmaur M, d'Amore ES, Joshi VV, Hata J, Roald B, Dehner LP. Revision of the International Neuroblastoma Pathology Classification: confirmation of favorable and unfavorable prognostic subsets in ganglioneuroblastoma, nodular. Cancer. 2003 Nov 15. 98(10):2274-81. [Medline].
- Plantaz D, Mohapatra G, Matthay KK, et al. Gain of chromosome 17 is the most frequent abnormality detected in neuroblastoma by comparative genomic hybridization. Am J Pathol. 1997 Jan. 150(1):81-9. [Medline].
- Plantaz D, Rubie H, Michon J, et al. The treatment of neuroblastoma with intraspinal extension with chemotherapy followed by surgical removal of residual disease. A prospective study of 42 patients--results of the NBL 90 Study of the French Society of Pediatric Oncology. Cancer. 1996 Jul 15. 78(2):311-9. [Medline].
- Powell JE, Esteve J, Mann JR, et al. Neuroblastoma in Europe: differences in the pattern of disease in the UK. SENSE. Study group for the Evaluation of Neuroblastoma Screening in Europe. Lancet. 1998 Aug 29. 352(9129):682-7. [Medline].
- Raney B, Ensign LG, Foreman J, et al. Renal toxicity of ifosfamide in pilot regimens of the intergroup rhabdomyosarcoma study for patients with gross residual tumor. Am J Pediatr Hematol Oncol. 1994 Nov. 16(4):286-95. [Medline].
- Raschella G, Cesi V, Amendola R, et al. Expression of B-myb in neuroblastoma tumors is a poor prognostic factor independent from MYCN amplification. Cancer Res. 1999 Jul 15. 59(14):3365-8. [Medline].
- Rascher W, Kremens B, Wagner S, et al. Serial measurements of neuropeptide Y in plasma for monitoring neuroblastoma in children. J Pediatr. 1993 Jun. 122(6):914-6. [Medline].
- Romansky SG. Neural crest aggregates in the human fetal adrenal gland. Laboratory Investigations. 1979. 40:9.
- Scanga DR, Martin WH, Delbeke D. Value of FDG PET imaging in the management of patients with thyroid, neuroendocrine, and neural crest tumors. Clin Nucl Med. 2004 Feb. 29(2):86-90. [Medline].
- Schmidt ML, Lal A, Seeger RC, et al. Favorable prognosis for patients 12 to 18 months of age with stage 4 nonamplified MYCN neuroblastoma: a Children's Cancer Group Study. J Clin Oncol. 2005 Sep 20. 23(27):6474-80. [Medline].
- Seeger RC, Siegel SE, Sidell N. Neuroblastoma: clinical perspectives, monoclonal antibodies, and retinoic acid. Ann Intern Med. 1982 Dec. ID - CA 22794/CA/NCI(6):873-84. [Medline].
- Shimada H, Ambros IM, Dehner LP, et al. Terminology and morphologic criteria of neuroblastic tumors: recommendations by the International Neuroblastoma Pathology Committee. Cancer. 1999 Jul 15. 86(2):349-63. [Medline].
- Shulkin BL, Shapiro B. Current concepts on the diagnostic use of MIBG in children. J Nucl Med. 1998 Apr. 39(4):679-88. [Medline].
- Shuster JJ, McWilliams NB, Castleberry R, et al. Serum lactate dehydrogenase in childhood neuroblastoma. A Pediatric Oncology Group recursive partitioning study. Am J Clin Oncol. 1992 Aug. 15(4):295-303. [Medline].
- Srivatsan ES, Murali V, Seeger RC. Loss of heterozygosity for alleles on chromosomes 11q and 14q in neuroblastoma. Prog Clin Biol Res. 1991. 366:91-8. [Medline].
- Tanaka T, Slamon DJ, Shimoda H, et al. Expression of Ha-ras oncogene products in human neuroblastomas and the significant correlation with a patient's prognosis. Cancer Res. 1988 Feb 15. 48(4):1030-4. [Medline].
- Tang XX, Evans AE, Zhao H, et al. High-level expression of EPHB6, EFNB2, and EFNB3 is associated with low tumor stage and high TrkA expression in human neuroblastomas. Clin Cancer Res. 1999 Jun. 5(6):1491-6. [Medline].
- Turkel SB, Itabashi HH. The natural history of neuroblastic cells in the fetal adrenal gland. Am J Pathol. 1974 Aug. 76(2):225-44. [Medline].
- Villablanca JG, Khan AA, Avramis VI, Reynolds CP. Hypercalcemia: a dose-limiting toxicity associated with 13-cis-retinoic acid. Am J Pediatr Hematol Oncol. 1993 Nov. - Reynolds CP(4):410-5. [Medline].
- Wang Q, Diskin S, Rappaport E, et al. Integrative genomics identifies distinct molecular classes of neuroblastoma and shows that multiple genes are targeted by regional alterations in DNA copy number. Cancer Res. 2006 Jun 15. 66(12):6050-62. [Medline].
- White PS, Maris JM, Sulman EP, et al. Molecular analysis of the region of distal 1p commonly deleted in neuroblastoma. Eur J Cancer. 1997 Oct. 33(12):1957-61. [Medline].
- Woods WG, Tuchman M, Robison LL, et al. A population-based study of the usefulness of screening for neuroblastoma. Lancet. 1996 Dec 21-28. 348(9043):1682-7. [Medline].
- Yamashiro DJ, Liu XG, Lee CP, et al. Expression and function of Trk-C in favourable human neuroblastomas. Eur J Cancer. 1997 Oct. 33(12):2054-7. [Medline].
- Yamashiro DJ, Nakagawara A, Ikegaki N, Liu XG, Brodeur GM. Expression of TrkC in favorable human neuroblastomas. Oncogene. 1996 Jan 4. 12(1):37-41. [Medline].
- Yanagisawa T, Newman A, Coley H, et al. BIRICODAR (VX-710; Incel): an effective chemosensitizer in neuroblastoma. Br J Cancer. 1999 Jun. 80(8):1190-6. [Medline].
Histologic subtypes of neuroblastoma. Top right panel, neuroblastoma: A monotonous population of hyperchromatic cells with scant cytoplasm. Bottom left panel, ganglioneuroblastoma: Increased schwannian stroma. Bottom right panel, ganglioneuroma: Mature ganglion cell with schwannian stroma.
CT scan of abdomen in a patient with a retroperitoneal mass arising from the upper pole of the left kidney and elevated urine catecholamines.
MRI of a left adrenal mass. The mass was revealed by fetal ultrasonography at 30 weeks' gestation. During infancy, the mass was found on the inferior pole of the left adrenal and was completely resected. Before surgery, the metastatic workup was negative. Surgical pathology service confirmed a diagnosis of neuroblastoma. After 3 years of follow-up care, no recurrence was observed.
A one-week-old neonate had abdominal ultrasonography for evaluation of projectile vomiting. A right adrenal mass (100% cystic) was an incidental finding. Evaluation of the mass by CT was consistent with an adrenal bleed (3.6 x 3.1 x 2.4 cc). The infant was followed at 2 weeks (2-dimensional size diminished to 1.5 x. 2.4 cm2 on ultrasonography) and then at 6 weeks to document that the adrenal bleed continued to involute. Urine catecholamines were normal.
Table. A Consensus Pretreatment Classification schema by the International Neuroblastoma Risk Group (INRG). This schema is based in the INRG stage, age, histologic category, tumor grade of differentiation, MYCN sastus, 11q-aberrations and DNA ploidy. A combination of these characteristics results in four risk groups noted in the last column: very low, low, intermediate and high risk, with the following 5 year EFS: >85%, >75%-85%, >50%-75%, and < 50%. These risk groups are distributed among the different stages and labeled alphabetically from A to R (without letters L and M to avoid confusion with the INRG stage notation). Notations in the table are as follow: L1, localized tumor confined to one body compartment; L2, locoregional tumor with presence of one or more risk factors defined radiologically; M, distant metastatic disease (except stage MS); MS, metastatic disease confined to skin, liver and/or bone marrow in children < 18 months of age. GN, ganglioneuroma; GNB, ganglioneuroblastoma; Amp, amplified; n/amp, not amplified. (Adapted from The International Neuroblastoma Risk Group (INRG) Classifications System: An INRG Task Force Report by Cohn, et al. Journal of Clinical Oncology 27(2):289-297, 2009).
- Table 1. Current COG Neuroblastoma Risk Stratification
Table 1. Current COG Neuroblastoma Risk Stratification
| Risk Group | Stage | Age | MYCN Amplification Status | Ploidy | Shimada |
| Low | 1 | Any | Any | Any | Any |
| Low | 2a/2b | Any | Non-amp | Any | Any |
| High | 2a/2b | Any | Amp | Any | Any |
| Intermediate | 3 | < 547d | Non-amp | Any | Any |
| Intermediate | 3 | ≥547d | Non-amp | Any | Favorable |
| High | 3 | Any | Amp | Any | Any |
| High | 3 | ≥547d | Non-amp | Any | Unfavorable |
| High | 4 | < 365d | Amp | Any | Any |
| Intermediate | 4 | < 365d | Non-amp | Any | Any |
| High | 4 | 365-547d | Amp | Any | Any |
| High | 4 | 365-547d | Any | Diploid | Any |
| High | 4 | 365-547 | Any | Any | Unfavorable |
| Intermediate | 4 | 365-547d | Non-amp | Hyper | Favorable |
| High | 4 | ≥547d | Any | Any | Any |
| Low | 4s | < 365d | Non-amp | Hyper | Favorable |
| Intermediate | 4s | < 365d | Non-amp | Diploid | Any |
| Intermediate | 4s | < 365d | Non-amp | Any | Unfavorable |
| High | 4s | < 365d | Amp | Any | Any |
